Hey CFD and modeling enthusiasts!
Today, I’m thrilled to share some exciting updates from my PhD journey at Aalto University. As some of you know, I started my PhD in Chemical Engineering back in 2023, focusing on liquid-liquid extraction, generously funded by Neste Company. Our latest manuscript is part of a series stemming from this work, diving into the fascinating world of liquid-liquid phase separation, especially in the presence of surfactants.
Unveiling the Role of Surfactants
In our recently published manuscript, we explore how surfactants influence phase separation and contribute to dispersion stability by forming stable microemulsions. For those who want to dive deeper into the fundamentals, feel free to revisit our previous blog post on liquid-liquid phase separation. You can access the code and additional details through: https://doi.org/10.1016/j.ces.2024.120558.
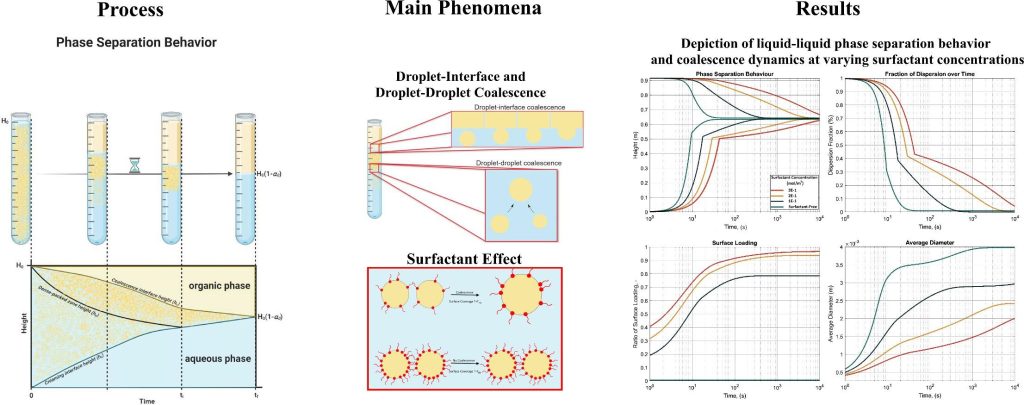
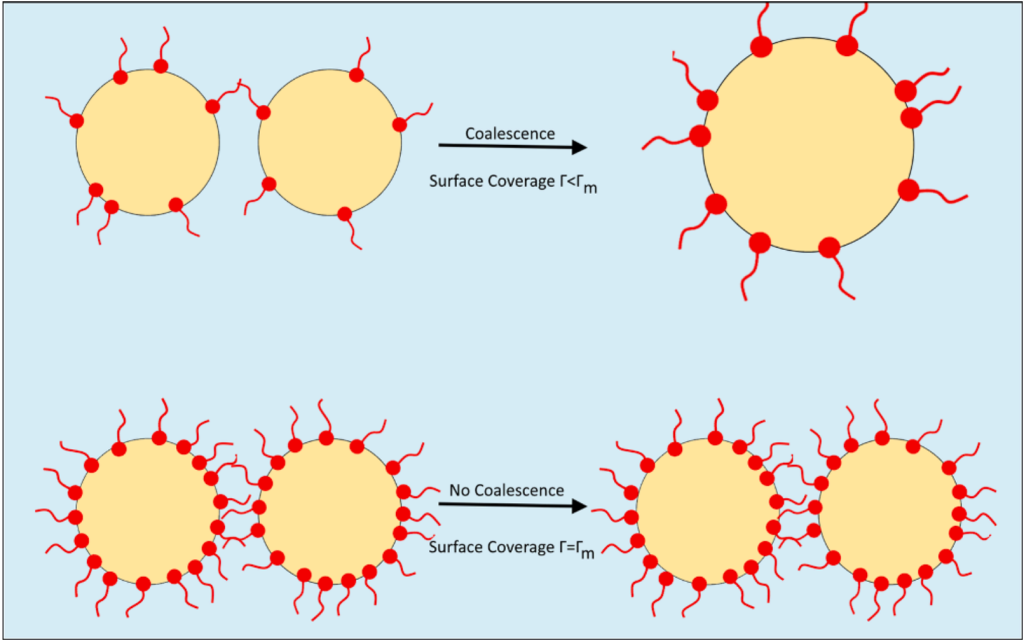
To put it simply, surfactants tend to accumulate at the interface between two immiscible fluids, such as oil and water. This surface accumulation creates a barrier that prevents coalescence, which is the merging of droplets. As highlighted in our earlier discussion, the two main coalescence processes in liquid-liquid phase separation are droplet-droplet (binary) coalescence and droplet-interface coalescence. Surfactants hinder these processes by adsorbing onto the interface, as illustrated below:
Modeling the Impact of Surfactants
A critical question arises: How do we model the effect of surfactants on phase separation? The key lies in understanding the adsorption process. Isotherms, like Langmuir or Frumkin, can model surfactant adsorption, illustrating how much of the available surfactant can adsorb onto the surface. When the surface is fully covered, coalescence is effectively halted.
But how do we measure the adsorption of a specific surfactant, such as Triton X-100, onto a surface? The answer is experimentation! By determining the amount of surfactant (in moles) that can sit at the interface, we can calculate its impact on the coalescence hindrance term.
Calculating Available Surface Area
Another crucial aspect is calculating the available surface area for surfactant adsorption. This is where population balance comes into play. Initially, we have a known population of the dispersion. By applying coalescence and breakage kernels, we can calculate how this population evolves over time, determining the radius and number of droplets in the dispersion. Knowing the number and diameter of the droplets, we can easily calculate the total surface area available for surfactant adsorption through multiplication.
Key Takeaways from Our Research
Our research, titled “Modeling Oil/Water Emulsion Separation in Batch Systems with Population Balances in the Presence of Surfactant,” offers valuable insights into the interplay between surfactants and liquid-liquid phase separation. However, it’s important to remember that while modeling and CFD provide powerful tools, they are not always the definitive solution for every challenge.
Stay curious, keep experimenting, and continue refining your simulations and models. The world of CFD and modeling is vast and full of exciting discoveries.
Until next time, stay sharp and keep simulating!