Abstract
Continuous Stirred Tank Reactors (CSTRs) play a pivotal role in the chemical and process industries due to their steady-state operational capabilities. This study delved into the influence of initial temperature on the dynamics of a CSTR system. Focusing on the reaction A+B→P, which follows a second-order rate law, the role of temperature in reaction kinetics, as described by the Arrhenius equation, was examined. The study explored the initial temperature impact on the reactant and product concentrations and the overall temperature by simulating the reactor’s behavior using MATLAB and varying the initial temperature. Key findings revealed that the initial temperature profoundly affects reaction rates, time to reach steady states, and the overall behavior of the reactor. Multiple steady states were observed in some cases, highlighting the complexities inherent in reactor dynamics. This research underscores the importance of understanding the implications of initial temperature, providing crucial insights for optimizing reactor performance, safety, and design strategies in industrial applications.
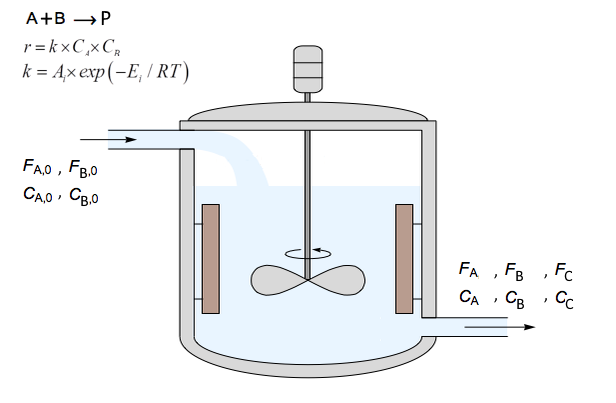
Introduction
Continuous Stirred Tank Reactors (CSTRs) are among the most common reactors in the chemical and process industries. Their versatility and capacity for steady-state operations make them particularly suitable for various reactions. In a CSTR, the reactants are continuously fed into the reactor while the products are continuously removed, ensuring a uniform composition and temperature throughout the reactor volume. CSTR reactor contrasts with other reactor types, such as batch reactors, where all reactants are loaded initially and left to react, and the products are removed at the end.
Various factors, including the type of reaction, rate constants, feed compositions, and initial conditions, can influence the dynamics of a CSTR. The present study focuses on the reaction A+B→P, which follows a second-order rate law. Given the critical role temperature plays in reaction kinetics, mainly as dictated by the Arrhenius equation, understanding the impact of initial reactor temperature on system dynamics is paramount.
This simulation study aims to delve deep into the reactor’s behavior by varying the initial temperature. By doing so, we seek to understand how this factor influences concentrations of reactants and products over time and the reactor’s overall temperature profile. Further, given the complex interplay of reaction kinetics, heat effects, and mass transfer, the intriguing possibility exists to observe multiple steady states under certain conditions.
Through this exploration, we aim to understand the fundamental behavior of our CSTR system under varying initial conditions and glean insights that can guide operational and design strategies for real-world applications.
Theoretical Background
CSTRs are a cornerstone in the realm of chemical engineering due to their ubiquity in many chemical processes. To understand the intricacies of their behavior, one must delve into the fundamental governing equations and principles underlying their operation. This section elucidates the theoretical framework pertinent to our CSTR study.
Reaction Kinetics
The reaction under consideration, A+B→P, is governed by a second-order rate law, mathematically represented as:
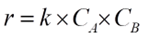
Where r is the rate of reaction, k is the rate constant, and CA and CB are the concentrations of reactants A and B, respectively. The Arrhenius equation defines the relationship between the rate constant k and temperature T:
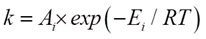
Here, Ai is the frequency factor or pre-exponential factor, Ei is the activation energy, and R is the universal gas constant. This equation underscores the sensitivity of reaction rates to temperature, an aspect that becomes pivotal in our study.
Material Balances
Overall mass balance for a CSTR can be written as:
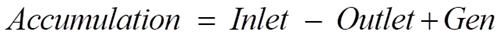
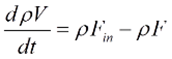
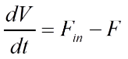
Where ρ denotes mixture density, F represents the volumetric flow rate, and V represents the reactor volume.
For any reactor, the dynamics of the components are determined by material balances.
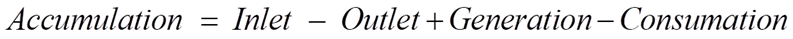
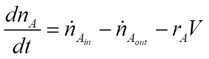
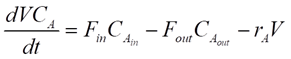
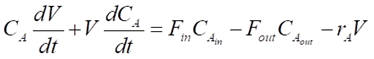
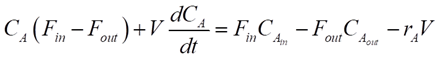
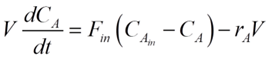
For our CSTR system, the balances for reactants A and B and product P are represented as:
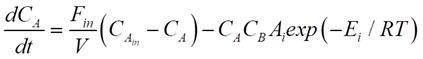
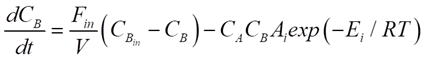
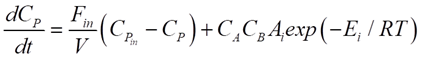
Where CA, CB and CP are A, B and P feed concentrations, respectively.
Energy Balance
Being an adiabatic system, the CSTR does not exchange heat with its surroundings. The energy dynamics are thus steered by the heat brought in by the feed, the heat taken out by the product, and the heat generated or absorbed by the reaction. Energy balance can be mathematically represented by:
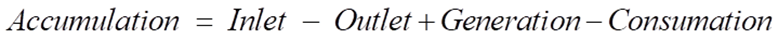
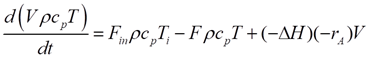
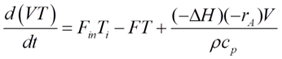
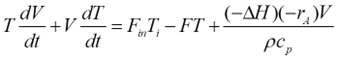
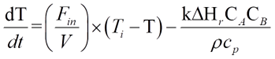
Ti is the feed temperature, and ΔHr signifies the reaction enthalpy. The reaction enthalpy dictates whether the reaction is endothermic (absorbs heat) or exothermic (releases heat), further influencing reactor temperature dynamics.
With this theoretical foundation, we are equipped to simulate the reactor’s behavior under various conditions, thereby unveiling patterns and insights pertinent to reactor operation and design.
Methodology
The methodology employed for this study revolves around using MATLAB as a simulation tool to investigate the behavior of the CSTR system under different initial conditions. The entire process can be broken down into various sequential steps, which are detailed below:
Description of the MATLAB Approach Used
MATLAB, renowned for its robust numerical solvers and versatile environment, was utilized to simulate the CSTR system dynamics. The system’s governing equations, derived from first reaction kinetics and energy balance principles, were solved numerically using the ode113 solver, an adaptive Runge-Kutta method ideal for stiff or non-stiff systems.
Model Setup: Definitions of Constants and Parameters
All relevant parameters and constants were defined to set up the reactor model:
- Feed concentrations: C_A0, C_B0, and C_P0.
- Reactor volume (V_R) and volumetric flow rate (V_f).
- Activation energy (E) and frequency factor (A).
- Reaction enthalpy (deltaH_r).
- Heat capacity (rho__c_p).
- Universal gas constant (R).
- Feed temperature (T0) and system initial temperature (Ti).
These parameters form the backbone of the simulations, providing necessary inputs for our differential equations.
ODE Function Description
The core function odefun was implemented to contain the differential equations governing the CSTR system’s behavior. Within this function:
- The reaction rate constant, k, was determined using the Arrhenius equation.
- The reaction rate, r, was computed using the second-order rate law.
- Material balances for components A, B, and P were formulated based on inlet and outlet flow rates and the reaction rate.
- An energy balance was derived considering the adiabatic nature of the reactor, heat introduced via the feed, and heat generated or absorbed due to the reaction.
Simulation Strategy: Varying the Initial Reactor Temperature
To investigate the CSTR dynamics under various conditions, the system’s initial temperature, Ti, was varied in a defined range (from 290 K to 400 K in increments of 10 K). For each iteration, the differential equations were solved over a specified time span using ode113. Also, results were plotted to visualize the concentrations of A, B, and P, alongside the reactor temperature as a function of time. The simulation outputs were saved as image and data CSV files for further analysis.
Plotting and Results Interpretation Methods
The results were visualized for each simulation corresponding to a specific initial temperature using MATLAB’s plotting functions. Each plot featured:
- Concentrations of components A, B, and P on the primary y-axis.
- Reactor temperature on the secondary y-axis, ensuring clear differentiation.
- Time (in hours) on the x-axis.
- A legend indicating each plotted variable for clarity.
- A grid for easy reference and title indicates the simulation’s initial temperature.
These plots visually represent the CSTR’s dynamic response, facilitating insights into how initial temperature influences reactor behavior.
Results and Discussion
Through the MATLAB simulation, an in-depth analysis of the reactor dynamics was performed by monitoring the variations in concentrations and temperature within the reactor vessel. The objective was to observe the system dynamics until a steady state was achieved.
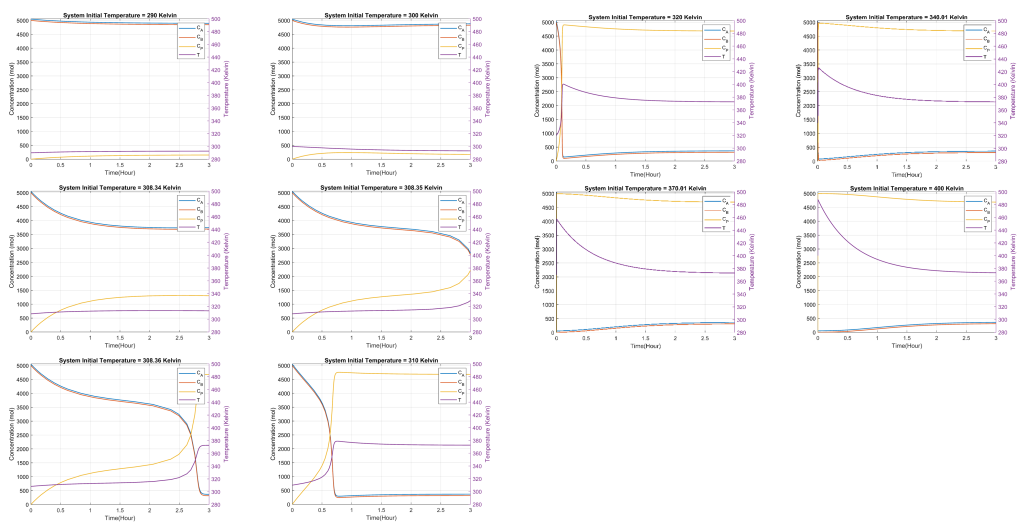
The reactor was assumed to be initially filled with an inert solvent for our investigation. The initial temperature, denoted as Ti, was varied in a range of 290-400 K. The goal was to study how this wide temperature span affects the reactor dynamics. Specifically, twelve different simulations were conducted, each differing in its initial reactor temperature.
The feed temperature, denoted as T0, remained consistent at 290 K throughout all simulations. The results were plotted in Figure 1 for each specific initial temperature, illustrating concentrations of components A, B and P and temperature.
Video 1 shows more different initial temperature:
On visual inspection of the plots, the following key observations emerged:
- Typically, an increase in temperature will increase the reaction rate, assuming the reaction is endothermic. This is due to the Arrhenius equation, which shows that the reaction rate constant (k) increases with increasing temperature. As Ti rises, the value of k would be higher at the beginning of the reaction, leading to a faster initial reaction rate. In the plots generated above, faster reaction rates should correspond to steeper initial slopes for the concentration curves. Also, it shows how the increase in Ti can lead to multiple steady states.
- Reaching a steady state means the system’s variables (concentrations and temperature) no longer change with time. The speed at which a steady state is reached can be affected by Ti. A higher initial temperature drives the system to its steady state faster.
- Since the reaction is exothermic (releases heat), the reaction will generate its own heat, potentially raising the system’s temperature. Starting with a higher Ti might mean the system reaches a maximum tolerable temperature faster, potentially slowing or stopping the reaction if it gets too high. In the plots, the temperature (T) curve plateau or even decreases after initially increasing, indicating the system reaches a maximum temperature due to the heat of the reaction.
- A faster reaction rate typically means products are formed more quickly. However, the total amount of product formed when the system reaches a steady state might not necessarily change with varying Ti. The initial temperature affects the speed of product formation but not the final product amount.
- The concentration of product P demonstrated intriguing behavior. With lower initial temperatures, the production rate gradually showed a standard behavior of reactants converting to products. However, upon reaching a certain threshold (around Ti = 308 K), there was a pronounced spike in the production of P. This implies that the reaction kinetics were significantly amplified, rapidly converting A and B to P.
- For the temperature dynamics, a clear trend was not always consistent across all simulations. However, a particular attention-grabbing behavior was noted post the threshold of Ti = 308 K, where an initial spike in temperature was observed, possibly due to the exothermic nature of the reaction and the sudden increase in reaction rate.
The initial temperature (Ti) can significantly influence the dynamics of a chemical reaction system. It can affect the reaction speed, the time taken to reach a steady state, and the system’s overall behavior. Careful control and understanding initial conditions, like temperature, are crucial for optimizing chemical processes and ensuring safety.
Conclusion
The study provided a comprehensive analysis of the behavior of a Continuous Stirred Tank Reactor (CSTR) system by varying the initial temperature. The findings demonstrate that initial temperature, Ti, significantly influences the system’s dynamics in terms of reaction speed, concentrations of reactants and products over time, and overall temperature profile. The importance of the Arrhenius equation was further solidified by observing the correlation between the initial temperature and the reaction rate. The significance of this study lies in its ability to offer insights crucial to the design and operation of CSTRs. In real-world applications, understanding the influence of initial temperature on system dynamics will aid in optimizing reactor performance, enhancing productivity, and ensuring operational safety. The intriguing observation of multiple steady states under specific conditions underscores the potential challenges and complexities in reactor operation and design.
I’m glad you’ve joined me on this journey through today’s topic. Thank you for taking the time to read and engage with my post. Your interest and support are what keep this blog alive and thriving. As a token of appreciation and to help you continue exploring and experimenting, I’ve made relevant files available for download from github. Feel free to use them as a resource for your own projects or learning. And remember, this simulation of ideas and creativity doesn’t have to end here – let’s keep the conversation going in the comments section. Stay curious and keep experimenting! 🔬🚀
